Product Description
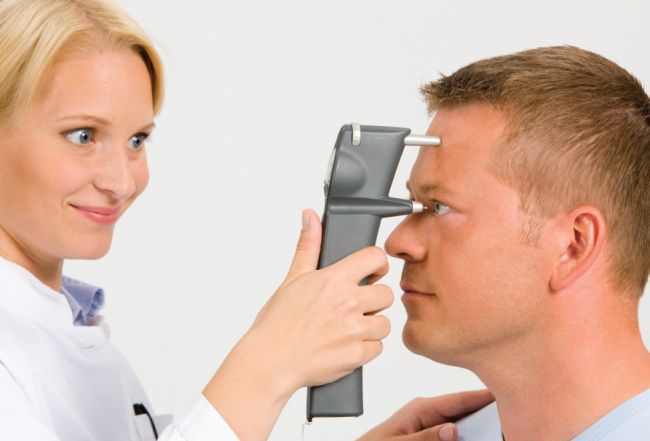
The iCare tonometer is based on a proven accurate measuring principle, in which a very light probe is used to make momentary and gentle contact with the cornea. The measurement is barely noticed by the patient and often does not even cause corneal reflex. The device not only makes IOP measuring a more pleasant experience on all patients, it is also an important break-through for succeeding with non-compliant patients (i.e. children and dementia patients). Requiring no drops, neither specialized skills for its use the quick and painless iCare tonometry has an important role in IOP screening programs of masses. The measurement is barely noticed by the patient and therefore suitable even for non-compliant patients, such as children and dementia patients. The easy usage without anesthesia and dynamic patient flow obtained by the iCare tonometer make it a very important instrument for ophthalmologists, optometrists, general practitioners, occupational health care and other medical personnel.
The iCare will forever change the way you measure IOP
No Drops, No Air, No Tears, No Pain…
Tonometry in your hands, without the sacrifice of patient comfort. Until now, IOP measuring meant patient discomfort using drops, anesthetic, or a puff of air. No longer do patients need to fear a routine IOP measuring. At the same time, iCare offers extraordinary accuracy, measuring within 1.2 units to the standard Goldman Tonometer.
Anyone can operate the iCare Tonometer, and it can be preformed on just about anyone. Designed to be portable, the iCare allows the technician sufficient IOP measuring on even non-compliant patients without compromising accuracy. With the handheld design, the iCare rotates to create an angle to accommodate patients lying horizontally, making it the perfect tonometer for youth or elderly patients who may not be ideal.
UNIQUE TECHNOLOGY ENABLES SAFE, PAINLESS AND HYGIENIC IOP MEASUREMENT
Icare rebound tonometer was developed to find a new, easy and patient-friendly method of measuring intraocular pressure. It is a major breakthrough obtained after a decade of development work by a Finnish MD, Mr. Antti Kontiola. The realization of the rebound method has involved a number of clinical test partners, including the Helsinki University Eye Clinic (Finland) in the human application, and the Mount Sinai Medical School (US) in the animal application.
The rebound technology is based on the rebound measuring principle, in which a very light-weight probe is used to make a momentary contact with the cornea. In the rebound technology, motion parameters of the probe are recorded during the measurement. An induction based coil system is used for measuring the motion parameters. An advanced algorithm combined with the state of the art software analyzes deceleration and the contact time of the probe while it touches the cornea. Deceleration and the contact time of the probe change as a function of IOP. In simple terms, the higher the IOP, the faster the probe decelerates and the shorter the contact time.
Glaucoma is progressive disease which causes permanent damages to
Papilla
Optic nerve
Optic field
Main risk factors of glaucoma
Increased IOP (= intra-ocular pressure)
Age
Family history of glaucoma
Exfoliation Syndrome
Myopia
Target of Glaucoma treatment
To prevent eye sight damage and blindness
So far the only treatment method is to lower IOP
Can gradually steal sight without any warning or symptoms
Incurable disease which can cause blindness if untreated
Blindness is ranked 3rd (after cancer + heart disease) as people’s major fear
Glaucoma causes 9 – 12 % of all blindness
Early detection is vital to stop the progress of the disease, but vision lost cannot be regained
Most important, and only method to screen masses of population is rapid IOP measuring
Here are some of the most Frequently Asked Questions about the iCare Tonometer:
IS THE ICARE MEASUREMENT PAINLESS?
Measurement is painless, the light-weight probe touches the cornea momentarily and some patients don’t even notice the measurement.
IS IT POSSIBLE TO HURT THE EYE WITH ICARE?
If the device is used according to the instructions given in user manual, the light-weight probe cannot cause any damage to the patient’s eye.
IS THE ICARE MEASUREMENT RESULT ACCURATE?
Several independent studies prove the accuracy of Icare readings. Extensive bench testing and clinical studies have also been performed to ensure the accuracy and repeatability of measurements.
WHY SIX MEASUREMENTS?
Six measurements are required to provide accurate measurement results by eliminating the variations caused by operator error and heart rate.
DOES ICARE TAKE INTO CONSIDERATION CCT?
Icare tonometer is calibrated for average CCT just like the Goldmann tonometer.
CAN ICARE BE USED WITH CONTACT LENSES?
Yes, but depending on the type of contacts the tonometer readings may be affected by the contact lens. Soft daily disposable lenses (and some soft 2-week or monthly lenses) affect the reading only slightly. Hard contact lenses have a significant effect on the reading and should not be worn when measuring the IOP with Icare tonometer. For most accurate measurement results use of any contact lenses is not recommended.
CAN ICARE BE USED AFTER EYE SURGERY?
Yes, but consider that some of surgeries e.g. LASIK and similar surgeries may change the thickness of the cornea (thinner cornea => lower /false IOP)
CAN ICARE BE USED WHEN THE EYE IS INFECTED?
Yes. Just remember that the probe used to measure the infected eye cannot be used again, even for measuring the non-infected eye of the same patient.
DOES THE CORNEAL SHAPE AND RADIUS INFLUENCE THE IOP READING TAKEN WITH ICARE?
No. The area of contact is so small, that the measurement is not influenced by these aspects.
CAN ANESTHESIA STILL BE USED?
Icare is designed to be used without applying topical anesthetic. For most accurate measurements, we recommend not to apply topical anesthetic when measuring with Icare tonometer. It is possible to measure anesthetized eye but the readings may be affected by the swelling caused by topical anesthetic.
IS IT POSSIBLE TO LOAD PROBE WRONG WAY?
No, it’s not possible to load the probe wrong way. The mechanical design of probe and probe base makes it possible to insert the probe in a correct way.
CAN SAME PROBE BE USED TO MEASURE BOTH EYES OF ONE PATIENT?
Yes, same probe can be used to measure both eyes of one patient if the patient’s eyes are healthy. If the patient has infection in one eye or if there is any doubt that one eye may havea disease that can be transferred from eye to eye, the healthy eye should be measured first. A probe used to measure an infected eye should never be usedto measure a healthy eye. If in doubt, always use a new probe.
HOW PROBES WILL BE DISPOSED OF?
Probes can be disposed of according to the hospital/clinic/practice standard regulations.
DOES ICARE REQUIRE CALIBRATION?
Icare tonometers do not require any maintenance calibration or regular service. The tonometers don’t have any parts that wear out, except for the probe base, which can become dusty or collect some particles that affect the probe movement. The probe base can be changed by the user as described in the user manual. The calibration can be checked if there’s doubt about the measurement results. In such case, you should contact your local dealer/ distributor.
If the tonometer requires service, for example has been dropped to the floor, you should also contact your local dealer/ distributor for further instructions.
Still can’t find what you’re looking for? Request Support.
ICARE TECHNICAL SERVICE, MAINTENANCE AND REPAIRS
ICARE REPAIR AND MAINTENANCE SERVICE
Icare warranty, repair and maintenance services are available via our global distributor network.
» Find your local Icare Distributor
When sending the Icare tonometer to the service, please fill in and print ICARE SERVICE REQUEST form, and attach it to your tonometer when shipped for service.
» Icare Service Request form
CHANGING AND CLEANING THE PROBE BASE
The probe base can be changed and cleaned by the user as per instructions (also available at your Icare tonometer user manual).
» Icare Probe Base Changing & Cleaning Instructions
CALIBRATION
Icare rebound tonometers do not require any maintenance calibration or regular service. The Icare tonometers don’t have any parts that wear out, except for the probe base, which can become dusty or collect some particles that affect the probe movement.
Nevertheless our technology does not require maintenance calibration, we do supply calibration service with calibration certificate. To have your Icare tonometer calibrated, please contact your local Icare distributor.
SPARE PARTS & ACCESSORIES
A range of spare parts for Icare tonometer is available via our distributors.
Icare Spare Parts & Accessories
Type: TA01i.
The device conforms to CE regulations.
Dimensions:
13 – 32 mm (W) * 45 – 80 mm (H) * 230 mm (L).
Weight:
155 g (without batteries), 250 g (4 x AA batteries).
Power supply: 4 x AA batteries.
Measurement range: 7-50 mmHg,
Display range: 0-99 mmHg
(IOP estimation beyond the measuring range).
Accuracy
(95% tolerance interval relative to manometry): ±1.2 mmHg (≤20 mmHg)
Repeatability (coefficient of variation): <8%.
Accuracy of display: 1.
Display unit: Millimeter mercury (mmHg).
There are no electrical connections from the tonometer to the patient.
The device has B-type electric shock protection.
Storage/transportation environment: Temperature +5 to +40 °C.
Rel. humidity 10 to 80% (without condensation)
US FDA approved 2007 (510(k) -number K063873)
Chinese SFDA approved 2008
Korean FDA 2010
Complies with:
Medical Device Directive 93/42/EEC
Canadian Medical Device Regulations





Greg – :
The iCare works and functions perfectly. Thanks for all your help and information!